A) Cysteine
B) Alanine
C) Tyrosine
D) Isoleucine
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the following reaction? (Assume workup to neutralize.) 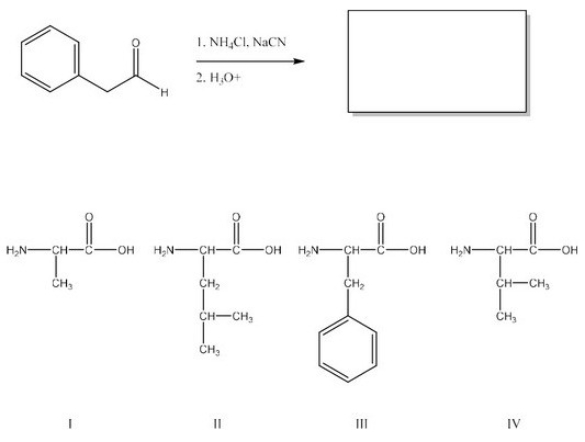
A) I
B) II
C) III
D) IV
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Amino acid synthesis is possible by all except one of the pathways listed.Which is not a synthetic pathway for amino acids?
A) Amination of malonic ester followed by hydrolysis and decarboxylation
B) Nucleophilic addition of NH3 to an aldehyde followed by addition of cyanide to the imine,and,finally,hydrolysis
C) SN2 reaction using an a-halo carboxylic acid with ammonia as the nucleophile
D) Reaction of NH4Cl and NaCN with an aldehyde followed by an acidic work-up
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Below is a proposed method for resolving a racemic mixture of (R) - and (S) -phenylalanine.Upon separation of the products,will this method produce (R) - or (S) -phenylalanine? 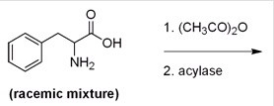
A) (R) -Phenylalanine is produced since the acylase only hydrolyzes the amides of L-amino acids.
B) (S) -Phenylalanine is produced since the acylase only hydrolyzes the amides of L-amino acids.
C) (R) -Phenylalanine is produced since the acylase only hydrolyzes the amides of D-amino acids.
D) (S) -Phenylalanine is produced since the acylase only hydrolyzes the amides of D-amino acids.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What amino acid is at the N-terminus of the following peptide? 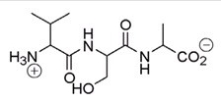
A) Valine
B) Serine
C) Alanine
D) Proline
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct structure for alanine at pH = 7? 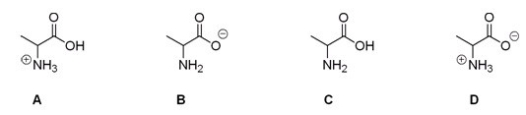
A) A
B) B
C) C
D) D
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why does the formation of salts of a racemic mixture of N-acetyl valine with a single enantiomer of a-methylbenzylamine enable the separation of the two enantiomers of N-acetyl valine?
A) They form amides.
B) They form diastereomers.
C) They form salts.
D) They form meso compounds.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the correct structure for the amino acid proline? 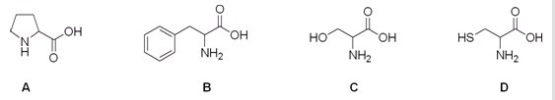
A) A
B) B
C) C
D) D
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the basic steps in the Merrifield peptide synthesis?
A) Coupling and hydrolysis
B) Hydrolysis and deprotection
C) Deprotection and hydrogenation
D) Coupling and deprotection
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the correct structure for serine? 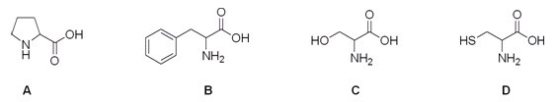
A) A
B) B
C) C
D) D
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following amino acids does not have an L-isomer?
A) Alanine
B) Valine
C) Glycine
D) Leucine
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product for the following reaction? 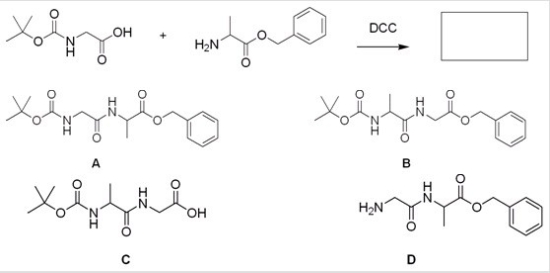
A) A
B) B
C) C
D) D
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the correct structure for the amino acid valine? 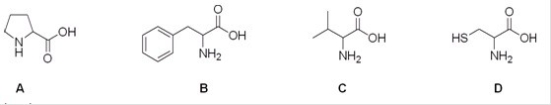
A) A
B) B
C) C
D) D
F) A) and B)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Which of the following correctly describes a protein?
A) a dipeptide; two amino acids joined together by one amide bond
B) a tripeptide; three amino acids joined together by two amide bonds
C) a polymer of more than 40 amino acids joined together by amide bonds
D) a polypeptide of any number of amino acids joined together by amide bonds
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is (are) globular protein(s) ?
A) Hemoglobin
B) Keratins
C) Collagens
D) Elastins
F) B) and D)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which of the following aldehydes would be a good starting material in the Strecker synthesis of phenylalanine? 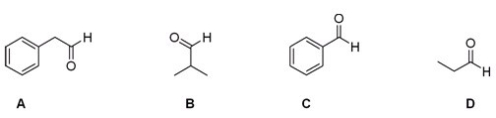
A) A
B) B
C) C
D) D
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Below is a 2D chromatogram that shows the separation of five amino acids.In this technique,the amino acid mixture is first separated by chromatography using a polar solvent system.Then the plate is rotated 90°,and the amino acids are further separated by electrophoresis.Identify the spots obtained from a mixture of Trp,Glu,Lys,Ile and Thr.A table with isoelectric points is included to aid in solving this problem. 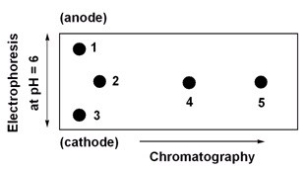 amino acid-pI value Trp-5.9
Glu-3.2
Lys-9.7
Ile-6.0
Thr-5.6
amino acid-pI value Trp-5.9
Glu-3.2
Lys-9.7
Ile-6.0
Thr-5.6
A) 1=Lys,2=Thr,3=Glu,4=Trp,5=Ile
B) 1=Glu,2=Thr,3=Lys,4=Trp,5=Ile
C) 1=Lys,2=Ile,3=Glu,4=Trp,5=Thr
D) 1=Glu,2=Ile,3=Lys,4=Trp,5=Thr
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the appropriate sequence of reaction conditions used to synthesize valine from acetamidomalonic ester? 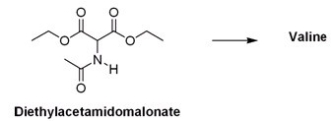
A) (1) NaOH; (2) (CH3) 2CHBr; (3) Cl2,H2O,heat
B) (1) NaOCH2CH3; (2) (CH3) 2CH2CHBr; (3) HCl,H2O,heat
C) (1) NaOCH2CH3; (2) (CH3) 2CHBr; (3) HCl,H2O,heat
D) (1) NaOH; (2) CH3CH2CH2Br; (3) HCl,H2O,heat
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What reagents would you use to convert acetaldehyde into alanine?
A) NH3
B) HCN
C) NaCN,NH4Cl
D) NaNH2
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the structure of the amino acid lysine? 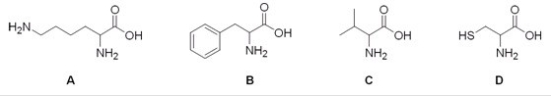
A) A
B) B
C) C
D) D
F) A) and D)
Correct Answer

verified
A
Correct Answer
verified
Showing 1 - 20 of 46
Related Exams